X chromosome inactivation is a vital biological phenomenon that ensures females do not express twice the amount of genes on the X chromosome compared to males. This intricate process, pivotal in understanding various X-linked disorders, has essential implications for the treatment of genetic diseases, including Fragile X Syndrome and Rett Syndrome. As researchers delve deeper into the mechanisms behind this silencing, breakthroughs are emerging that hint at potential gene therapy strategies for affected individuals. By unlocking the secrets of X chromosome inactivation, scientists aim to not only comprehend its role in genetic regulation but also pave the way for innovative therapies targeting mutations that cause debilitating conditions. The journey toward effective treatments could soon transform the lives of those battling genetic challenges linked to this critical chromosome.
The process of X chromosome silencing plays a crucial role in ensuring balanced gene expression in females, who possess two copies of the X chromosome. This chromosomal inactivation is more than just a biological curiosity; it carries significant weight in the context of various genetic disorders, including conditions that are commonly associated with X-linked mutations. Recent studies have illuminated the pathway to mitigating issues related to these genetic diseases, presenting new avenues for therapeutic interventions. By understanding how this chromosomal mechanism functions, scientists are laying the groundwork for targeted gene therapy solutions that can potentially alleviate the burdens of disorders like Fragile X Syndrome and Rett Syndrome. As researchers continue to unveil the complexities of this process, the future of genetics looks promising, particularly in the pursuit of effective treatments for those affected.
Understanding X Chromosome Inactivation and Its Role in Genetic Diseases
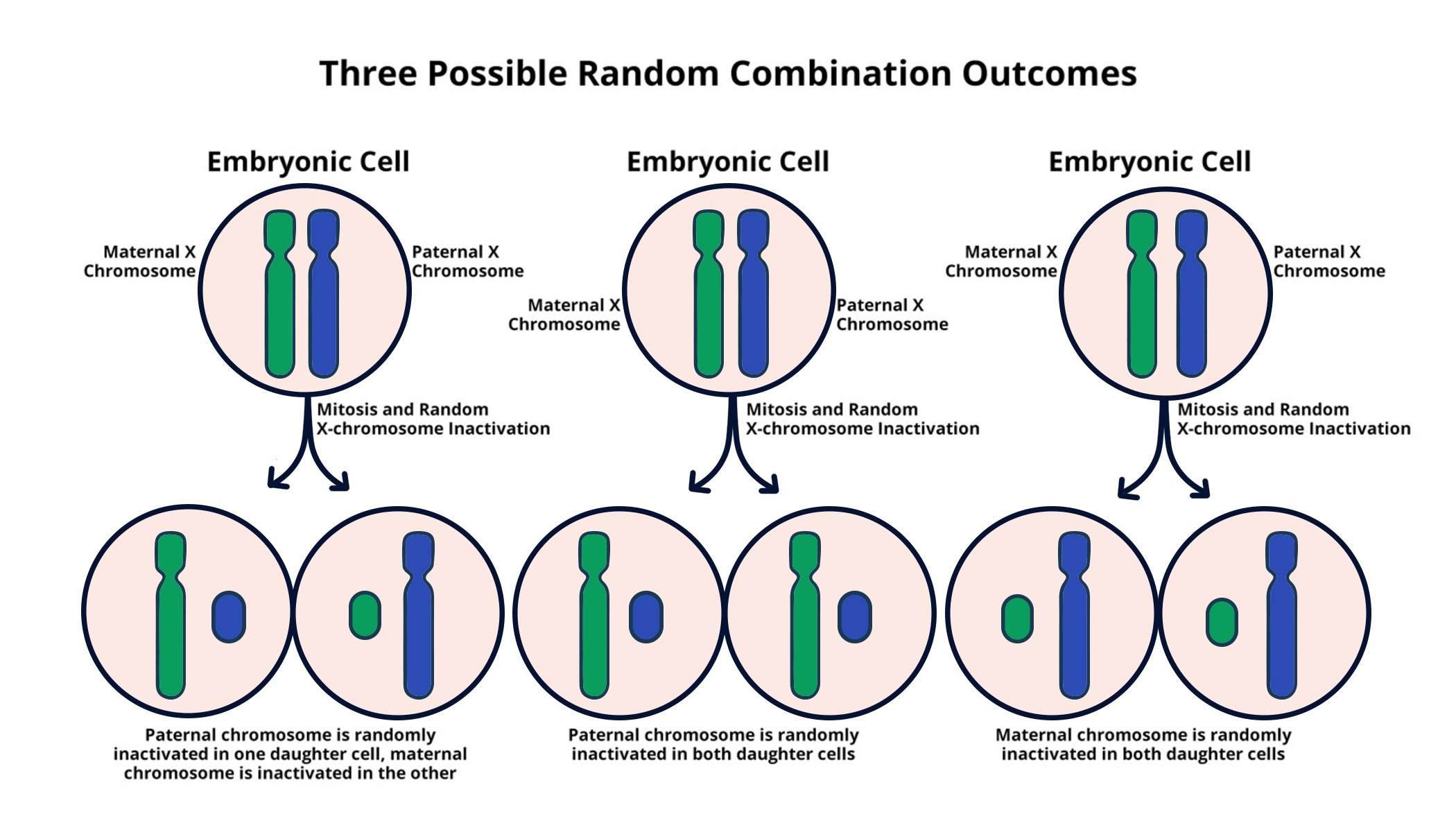
X chromosome inactivation (XCI) is a crucial biological process that occurs in female mammals, whereby one of the two X chromosomes is randomly silenced to ensure equal gene dosage between males and females. This process is vital for maintaining cellular homeostasis and plays a significant role in understanding various X-linked disorders. Genetic diseases such as Fragile X Syndrome and Rett Syndrome stem from mutations on the X chromosome, emphasizing the importance of XCI not only in basic biology but also in the potential development of therapies. The intricate mechanism behind XCI, particularly how Xist RNA interacts with chromosomal structures, provides insight into how mutations can be isolated and targeted for therapeutic interventions.
Recent findings suggest that the surrounding gelatinous substance, often referred to as the chromatin environment, plays a pivotal role in X chromosome inactivation. By modifying its biophysical properties, Xist allows for a more effective silencing of potentially harmful gene expression. Understanding how this process operates at the molecular level can lead to innovative gene therapy strategies aimed at reactivating silenced genes associated with genetic diseases, thereby offering hope for individuals affected by conditions linked to X-linked disorders.
The implications of X chromosome inactivation extend beyond mere genetic regulation; they also pave the way for novel therapeutic approaches. For example, harnessing the ability to manipulate X inactivation could provide a pathway to addressing Fragile X Syndrome and Rett Syndrome. Researchers are exploring ways to ‘unsilence’ the dormant X chromosome to restore function to crucial genes that are otherwise suppressed due to inactivation. This could transform treatment paradigms for those afflicted by such genetic conditions, offering a more targeted and effective means of management without introducing the complications that often accompany broader gene therapies.
Potential Therapies for Fragile X Syndrome and Rett Syndrome through Gene Therapy
The advancements made in our understanding of X chromosome inactivation open up exciting avenues for gene therapy aimed specifically at Fragile X Syndrome and Rett Syndrome. Fragile X Syndrome, which is the most common inherited form of intellectual disability, is caused by a mutation in the FMR1 gene located on the X chromosome. By leveraging knowledge of XCI, researchers are now investigating methodologies to reactive the silenced FMR1 gene in affected individuals. Such gene therapy could lead to remarkable improvements in cognitive function and behavior, significantly enhancing the quality of life for patients and their families.
Similarly, Rett Syndrome, which primarily affects girls and leads to severe neurodevelopmental issues, also presents a target for innovative gene therapy. Current research is focusing on the possibility of reactivating the MECP2 gene, which is often mutated or inactivated during development. By understanding how Xist interacts with chromatin to facilitate XCI, scientists believe they can devise strategies to restore the functionality of these crucial genes, thus potentially reversing the effects of these debilitating conditions. The promise of targeted gene therapy for these X-linked disorders underscores the necessity of understanding X chromosome biology.
Gene therapies aimed at X-linked disorders face unique challenges due to the intricacies of gene expression regulation through X chromosome inactivation. Developing safe and effective ways to deliver these therapies, whether through viral vectors or other means, is crucial to ensure that we can properly address the underlying genetic causes of Fragile X and Rett Syndromes. Moreover, ongoing safety studies are fundamental in determining the long-term effects of such therapies, ensuring that while we aim to reactivate harmful mutations, we do not inadvertently affect healthy genes on the X chromosome.
The path toward clinical trials is gaining momentum as research continues to unpack the mystery of XCI and its implications for gene therapy. As knowledge advances, the possibility of translating these basic biological principles into tangible therapies grows stronger. By honing in on specific mutations and understanding their regulation through X inactivation mechanisms, the future of treating genetic diseases linked to the X chromosome looks promising. This potential offers hope not only for current patients but also for future generations facing similar genetic challenges.
The Challenges of X-linked Disorders and Future Research Directions
Despite the advancements in understanding X chromosome inactivation, challenges remain in addressing the complexities of X-linked disorders. Conditions such as Fragile X Syndrome and Rett Syndrome are not only genetically intricate but also involve multiple pathways and cellular mechanisms that complicate treatment approaches. The variability in symptoms and severity among affected individuals makes it difficult to formulate a one-size-fits-all therapy. Specialized research is needed to tailor treatments based on the specific mutations and the degree of XCI in individual patients. Continued investigation into the nuances of genetic expression and silencing could unveil more personalized treatment modalities.
Future research should also focus on refining the techniques used in gene therapy to restore function to inactivated genes. This includes optimizing the delivery systems utilized to carry therapeutic genes into cells, ensuring maximum uptake and gene expression while minimizing potential side effects. Additionally, the community must explore the ethical implications of gene therapy, particularly relating to germline modifications, to ensure responsible application of these promising technologies without unintended consequences.
As we move forward, it is essential to cultivate a multidisciplinary approach in the research of X-linked disorders, integrating insights from genetics, molecular biology, and clinical practice. Collaborations between laboratories, hospitals, and biotechnology firms can accelerate the translation of research findings into real-world applications. Furthermore, patient involvement and advocacy will be vital in shaping the focus of research on X-linked disorders, ensuring that the needs and voices of affected individuals guide therapeutic development. With continued commitment and innovation, we stand at a pivotal threshold in the quest to unlock the mysteries of X chromosome inactivation and its potential for transforming the landscape of genetic disease treatments.
The Role of Xist RNA in X Chromosome Inactivation
Xist RNA is a fundamental player in the process of X chromosome inactivation (XCI), acting as a critical regulator that initiates the silencing of one of the X chromosomes in female mammals. When Xist is expressed, it coats the X chromosome that will be inactivated, altering its chromatin structure and ultimately leading to gene silencing. This process is essential not only for dosage compensation between sexes but also for understanding various genetic diseases linked to the X chromosome. The intricate mechanics of how Xist interacts with the molecular landscape of chromatin provides a promising target for therapies aimed at genetic disorders such as Fragile X Syndrome and Rett Syndrome, where mutations on the X chromosome play significant roles.
Recent studies have identified the interactions between Xist and the chromatin environment, often described metaphorically as
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to genetic diseases?
X chromosome inactivation is a biological process where one of the two X chromosomes in female cells is randomly inactivated to prevent a double dose of gene product. This mechanism is crucial as mutations on the X chromosome can lead to genetic diseases like Fragile X Syndrome and Rett Syndrome. By silencing one X chromosome, the effects of these mutations can be mitigated, making X chromosome inactivation a key target for therapeutic strategies in treating X-linked disorders.
How does X chromosome inactivation impact conditions like Fragile X Syndrome?
Fragile X Syndrome is an X-linked disorder caused by mutations on the X chromosome. X chromosome inactivation plays a significant role in this condition because it may render the healthy gene on the inactivated X chromosome unavailable for use. Therapies aimed at unsilencing these genes could potentially alleviate symptoms of Fragile X Syndrome by promoting the expression of healthy genes that are otherwise inactive.
What role does Xist play in X chromosome inactivation and genetic therapies?
Xist is an RNA molecule that initiates X chromosome inactivation by modifying the surrounding chromosomal environment, likened to a gelatinous substance. This process is essential for ensuring that one X chromosome in females is silenced. In gene therapy, harnessing Xist could help in unsilencing mutated genes linked to conditions like Fragile X Syndrome and Rett Syndrome, offering potential avenues for treatment and improved patient outcomes.
Can X chromosome inactivation be manipulated to develop therapies for X-linked disorders?
Yes, recent research suggests that manipulating X chromosome inactivation could lead to innovative therapies for X-linked disorders. By targeting the silencing mechanism, researchers can potentially reactivate genes on the inactivated X chromosome, allowing for expression of healthy genes, which is particularly beneficial for conditions like Fragile X Syndrome and Rett Syndrome.
What advancements have been made in using X chromosome inactivation in gene therapy?
Advancements include the development of methods to unsilence inactivated X chromosomes in isolated cells. Researchers, particularly in Jeannie T. Lee’s lab, are optimizing these techniques to potentially treat genetic diseases like Fragile X Syndrome and Rett Syndrome. Ongoing studies aim to evaluate the safety and efficacy of these approaches, with the hope of advancing them into clinical trials.
Why is X chromosome inactivation crucial for understanding genetic diseases?
Understanding X chromosome inactivation is crucial because it reveals how females manage the dosage of X-linked genes, which may harbor mutations linked to various genetic diseases. This knowledge provides insights into potential treatments for these conditions, as manipulating the inactivation process could restore proper gene function for disorders such as Fragile X Syndrome and Rett Syndrome.
Are there potential side effects of manipulating X chromosome inactivation for gene therapy?
While strategies to manipulate X chromosome inactivation aim to target mutated genes while sparing healthy ones, the potential for side effects remains. Current research indicates that unsilencing mechanisms could restore function to specific genes with minimal impact on surrounding healthy genes. Ongoing studies are focused on understanding these dynamics to ensure safety and efficacy in treatments for X-linked disorders.
How does X chromosome inactivation vary between males and females in relation to genetic disorders?
Males possess only one X chromosome, making them susceptible to diseases like Fragile X Syndrome if that chromosome carries a mutation. In contrast, females have two X chromosomes, so one can be inactivated, potentially masking the effects of a mutation on the other. This difference is vital for understanding the inheritance and manifestation of genetic disorders linked to X chromosome mutations.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation | The process where one of the two X chromosomes in females gets inactivated to ensure normal gene expression. |
| Research Background | Conducted by Jeannie T. Lee’s lab at Mass General, exploring molecular mechanisms of X chromosome inactivation. |
| Role of Xist RNA | Xist plays a crucial role in modifying the surrounding ‘Jell-O’ substance, facilitating the inactivation of the X chromosome. |
| Chromosomal Silencing Mechanism | ‘Jell-O’ around chromosomes allows Xist and associated molecules to easily access and inactivate the X chromosome. |
| Therapeutic Potential | Potential treatments for disorders like Fragile X Syndrome and Rett Syndrome by freeing inactivated X chromosomes. |
| Future Research | Ongoing optimization of therapeutic approaches, alongside safety studies, with aspirations for clinical trials. |
| Unresolved Questions | Still exploring why freeing inactivated genes doesn’t affect healthy genes on the X chromosome. |
Summary
X chromosome inactivation is a pivotal biological process that helps maintain gene balance between males and females. Recent research reveals essential mechanisms driving this process, highlighting the potential for innovative therapies for genetic disorders linked to the X chromosome. The work led by Jeannie T. Lee demonstrates that understanding X chromosome inactivation not only enriches our knowledge but also opens up avenues for treating conditions like Fragile X and Rett syndromes. As research continues, we may see significant breakthroughs that could transform the lives of those with X-linked genetic disorders.